Qhov Tseem Ceeb Qhov Sib Txawv - Muaj Zog Ligand vs Tsis Muaj Zog Ligand
Ib lub ligand yog ib qho atom, ion, los yog ib lub molecule uas pub los yog faib ob ntawm nws cov electrons los ntawm kev sib koom ua ke covalent daim ntawv cog lus nrog lub hauv paus atom lossis ion. Lub tswv yim ntawm ligands yog tham nyob rau hauv kev sib koom tes chemistry. Ligands yog hom tshuaj uas koom nrog hauv kev tsim cov complexes nrog hlau ions. Li no, lawv tseem hu ua complexing agents. Ligands tuaj yeem yog Monodentate, bidentate, tridentate, thiab lwm yam raws li qhov sib txawv ntawm lub ligand. Denticity yog tus naj npawb ntawm pab pawg neeg muaj nyob hauv ligand. Monodentate txhais tau hais tias ligand tsuas muaj ib pab neeg pub dawb xwb. Bidentate txhais tau tias nws muaj ob pab pawg pub dawb rau ib qho ligand molecule. Muaj ob yam loj ntawm ligands categorized raws li siv lead ua teb txoj kev xav; muaj zog ligands (los yog muaj zog teb ligands) thiab tsis muaj zog ligands (los yog tsis muaj zog ligands). Qhov sib txawv tseem ceeb ntawm cov ligands muaj zog thiab tsis muaj zog ligands yog tias kev sib cais ntawm orbitals tom qab khi rau ib qho chaw muaj zog ligand ua rau muaj qhov sib txawv ntawm qhov siab dua thiab qis zog orbitals qhov sib cais ntawm orbitals tom qab khi rau qhov tsis muaj zog ligand ua rau qis dua. nruab nrab ntawm qib siab thiab qis zog orbitals.
Crystal Field Theory yog dab tsi?
Crystals teb txoj kev xav tuaj yeem piav qhia raws li tus qauv uas yog tsim los piav qhia txog kev tawg ntawm degeneracies (electron plhaub ntawm lub zog sib npaug) ntawm electron orbitals (feem ntau yog d lossis f orbitals) vim qhov hluav taws xob zoo li qub tsim los ntawm ib puag ncig. anion los yog anions (los yog ligands). Qhov kev xav no feem ntau siv los qhia txog tus cwj pwm ntawm kev hloov pauv hlau ions complexes. Qhov kev xav no tuaj yeem piav qhia cov khoom sib nqus, xim ntawm kev sib koom ua ke, hydration enthalpies, thiab lwm yam.
Theory:
Kev sib cuam tshuam ntawm cov hlau ion thiab ligands yog qhov tshwm sim ntawm kev nyiam ntawm cov hlau ion nrog cov nqi zoo thiab cov nqi tsis zoo ntawm cov hluav taws xob tsis sib xws ntawm cov ligand. Qhov kev xav no feem ntau yog ua raws li cov kev hloov pauv tshwm sim hauv tsib degenerated electron orbitals (ib lub atom hlau muaj tsib d orbitals). Thaum ib lub ligand los ze rau ntawm cov hlau ion, cov electrons uas tsis tau ua ke yog ze rau qee qhov d orbitals dua li ntawm lwm cov d orbitals ntawm cov hlau ion. Qhov no ua rau poob ntawm degeneracy. Thiab tseem, cov electrons nyob rau hauv lub d orbitals repel cov electrons ntawm lub ligand (vim hais tias ob qho tib si tsis zoo them). Yog li cov d orbitals uas nyob ze rau lub ligand muaj zog ntau dua li lwm cov d orbitals. Qhov no ua rau kev sib cais ntawm d orbitals rau hauv siab zog d orbitals thiab qis zog d orbitals, raws li lub zog.
Qee yam cuam tshuam rau qhov kev sib cais no; Cov xwm txheej ntawm cov hlau ion, lub xeev oxidation ntawm hlau ion, kev npaj ntawm ligands nyob ib ncig ntawm lub hauv paus hlau ion thiab qhov xwm ntawm ligands. Tom qab kev sib cais ntawm cov d orbitals raws li lub zog, qhov sib txawv ntawm qhov siab thiab qis zog d orbitals yog hu ua crystal-field splitting parameter (∆ octrau octahedral complexes).
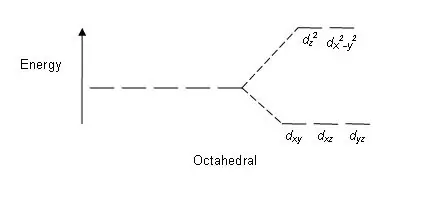
Daim duab 01: Sib faib qauv hauv Octahedral Complexes
Splitting qauv: Txij li muaj tsib d orbitals, qhov sib cais tshwm sim hauv qhov piv ntawm 2: 3. Nyob rau hauv octahedral complexes, ob lub orbitals yog nyob rau hauv lub siab zog theem (collectively hu ua 'eg'), thiab peb orbitals nyob rau hauv qis zog theem (collectively hu ua t2g). Nyob rau hauv tetrahedral complexes, qhov opposite tshwm sim; peb lub orbitals yog nyob rau hauv qib siab zog thiab ob nyob rau hauv qis zog theem.
Hnub Ligand yog dab tsi?
Ib lub ligand muaj zog lossis muaj zog ua teb ligand yog ligand uas tuaj yeem ua rau muaj kev sib cais ntau dua. Qhov no txhais tau hais tias, kev khi ntawm ib qho chaw muaj zog ligand ua rau muaj qhov sib txawv ntawm qhov siab dua thiab qis zog orbitals. Piv txwv xws li CN– (cyanide ligands), NO2– (nitro ligand) thiab CO (carbonyl ligands).
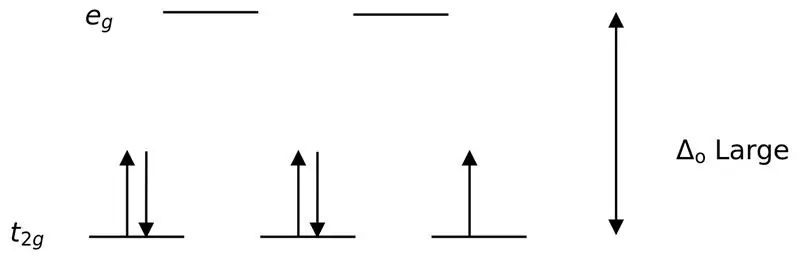
Daim duab 02: Tsawg Spin Spliting
Nyob rau hauv kev tsim cov complexes nrog cov ligands, thaum xub thawj, lub zog qis orbitals (t2g) yog tag nrho cov electrons ua ntej sau mus rau lwm yam siab zog orbitals (xws li). Cov complexes tsim nyob rau hauv txoj kev no yog hu ua "low spin complexes".
Tsis muaj zog Ligand yog dab tsi?
Ib lub ligand tsis muaj zog lossis tsis muaj zog ligand yog lub ligand uas tuaj yeem ua rau qis qis qis qis. Qhov no txhais tau hais tias, kev khi ntawm qhov tsis muaj zog ligand ua rau qhov sib txawv ntawm qhov siab dua thiab qis zog orbitals.
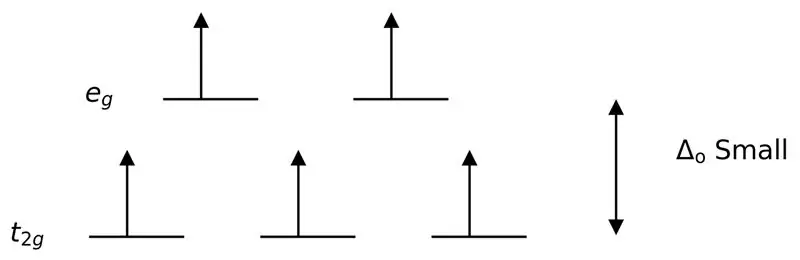
Daim duab 3: Siab Spin Spliting
Nyob rau hauv rooj plaub no, txij li qhov sib txawv qis ntawm ob lub orbital theem ua rau repulsions ntawm electrons nyob rau hauv cov qib zog, lub zog siab dua orbitals tau yooj yim sau nrog electrons thaum piv rau cov uas tsis muaj zog orbitals. Cov complexes tsim nrog cov ligands yog hu ua "high spin complexes". Piv txwv ntawm cov ligands tsis muaj zog muaj xws li I– (iodide ligand), Br– (bromide ligand), thiab lwm yam.
Qhov txawv ntawm Strong Ligand thiab Weak Ligand yog dab tsi?
Strong Ligand vs Weak Ligand |
|
| Ib lub ligand muaj zog lossis lub zog ligand yog lub ligand uas tuaj yeem ua rau muaj kev sib cais ntawm cov crystal ntau dua. | Ib lub ligand tsis muaj zog lossis tsis muaj zog ligand yog lub ligand uas tuaj yeem ua rau qis qis qis qis. |
| Theory | |
| Qhov kev sib cais tom qab khi ib qho chaw muaj zog ligand ua rau muaj qhov sib txawv ntawm qhov siab dua thiab qis zog orbitals. | Kev sib cais ntawm orbitals tom qab khi ib qho chaw tsis muaj zog ligand ua rau qhov sib txawv ntawm qhov siab dua thiab qis zog orbitals. |
| Cov complexes tsim muaj zog teb ligands hu ua "tsawg spin complexes". | Cov complexes tsim nrog tsis muaj zog teb ligands hu ua "siab spin complexes". |
Summary – Strong Ligand vs Weak Ligand
Cov ligands muaj zog thiab tsis muaj zog ligands yog anions lossis molecules uas ua rau tawg ntawm d orbitals ntawm cov hlau ion rau hauv ob theem zog. Qhov sib txawv ntawm cov ligands muaj zog thiab tsis muaj zog ligands yog tias qhov kev sib cais tom qab khi ib qho chaw muaj zog ligand ua rau muaj qhov sib txawv ntawm qhov siab dua thiab qis zog theem orbitals whereas lub splitting ntawm orbitals tom qab khi ib tug qaug zog teb ligand ua rau qhov sib txawv ntawm qhov siab dua thiab qis dua. lub zog theem orbitals.







